Preventing InfusAte injuries Throughout a Child's Hospitalisation (PATCH): A Type 1 Hybrid Randomised Controlled Trial
Implementing the most technologically advanced monitoring available for common healthcare-associated harm, improving the experiences of babies and families throughout their hospital stay.
Australian New Zealand Clinical Trials Registry: ACTRN1262300056184
Background
 Over 180,000 infants (<1 year of age) are admitted to Australian hospitals annually, and each is at risk of serious unintentional healthcare-associated iatrogenic injuries (e.g., extravasations, infections). Of the 180,000 infants admitted to Australian hospitals annually, ~60% (108,000) acquire an IV during their stay and our studies have demonstrated 33-45% of these IVs fail prior to treatment completion. This is commonly caused by damage to the vein where the IV is placed, resulting in the infusate fluid pooling in the tissue, rather than being administered into the bloodstream.
Over 180,000 infants (<1 year of age) are admitted to Australian hospitals annually, and each is at risk of serious unintentional healthcare-associated iatrogenic injuries (e.g., extravasations, infections). Of the 180,000 infants admitted to Australian hospitals annually, ~60% (108,000) acquire an IV during their stay and our studies have demonstrated 33-45% of these IVs fail prior to treatment completion. This is commonly caused by damage to the vein where the IV is placed, resulting in the infusate fluid pooling in the tissue, rather than being administered into the bloodstream.
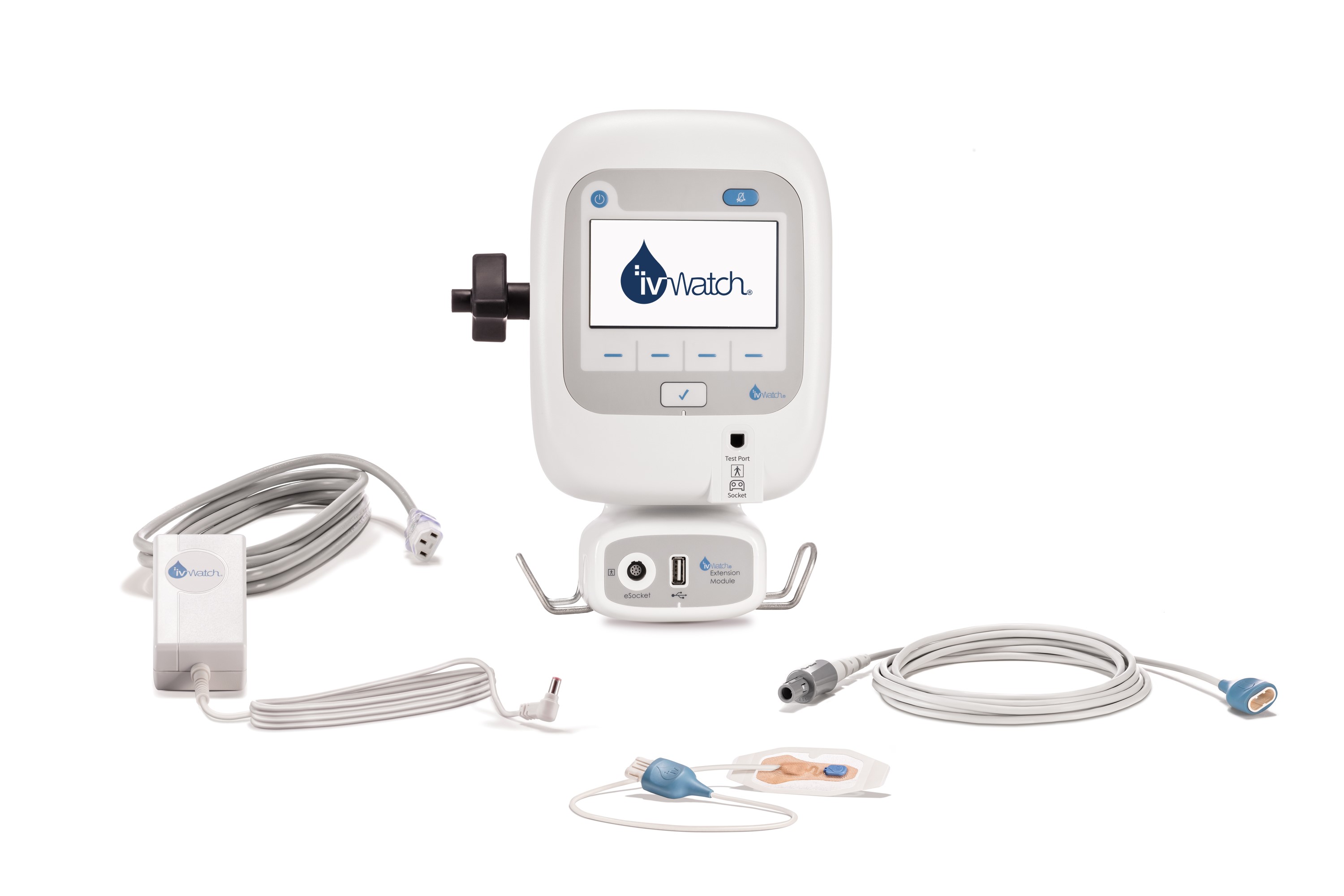
A potential solution to improve the detection of extravasations is an IV biosensor, such as ivWatch®. The TGA-approved, IV biosensor consists of a patient monitor, a reusable electronic cable, and a sterile disposable sensor secured to the patient’s skin adjacent to the IV site. The IV biosensor continuously monitors the optical properties of tissue near the IV site and provides audible and visual alarms when tissue fluid volume changes.
This is a multi-site, superiority Type-1 hybrid randomised controlled trial (RCT). A 2-arm, superiority, effectiveness RCT will test the effectiveness, and explore the value and implementation contexts, of an IV biosensor, compared to standard care, to detect extravasations and prevent extravasation injury. Concurrently, a mixed methods study will assess the uptake and attitudinal barriers and facilitators for implementation. The hybrid RCT will recruit and follow up across two tertiary-referral hospitals (Queensland Children’s Hospital (Brisbane), Royal Brisbane and Women’s Hospital (Brisbane) and one secondary-referral hospital (Sunshine Coast University Hospital (Sunshine Coast)) over a two-year period.
Investigators
- Professor Amanda Ullman
- Professor Nicole Marsh
- Professor Martha A.Q. Curley
- Professor Fiona M Coyer
- Professor Mark Davies
- Professor Samantha Keogh
- Associate Professor Lauren Kearney
- Associate Professor Craig McBride
- Ms Tricia M Kleidon
- Dr Deanne August
- Ms Mari Takashima
- Dr Hui (Grace) Xu
- Dr Roni Cole
- Professor Robert Ware
- Professor Joshua Byrnes
- Dr Clare Thomas
- Dr Sarfaraz Rahiman
- Dr Halley Ruppel
- Dr Christopher Bonafide
- Professor Brigid Gillespie
- Associate Professor Callan Battley
- Ms Rebecca Doyle
- Ms Victoria Gibson
- Ms Linda Nyugen
- Ms Anna Dubrovsky
- Ms Toni Day
Partner organisations
- University of Queensland
- Children's Health Queensland Hospital and Health Service
- Sunshine Coast University Hospital
- Royal Brisbane and Women’s Hospital
- Association for Wellbeing of Children in Healthcare, Clinical Excellence Queensland
- QUT
- Griffith University
This project has received funding from the NHMRC’s 2022 Medical Research Future Fund Clinician Researchers – Nurses, Midwives and Allied Health Grant.
Visit the PATCH Resources Page for more information.
